
 APK Android
APK Android
 IOS 64Bit
IOS 64Bit
 IOS 32Bit
IOS 32Bit
Free Test ID
ios 9+ install guide








About - 918Kiss
Previously, choosing an online casino with amazing slot games was easy. But now, the market is sprawling with every other casino. That is why we are here to cater to your needs and ensure you get more than you pay.
The casino in our platter today is 918Kiss, one of the most trustworthy casinos in Malaysia & Singapore. Since its inception, 918Kiss has aimed to become a reliable source for online gambling. Today, it is home to high-betting slot games with lucrative offers. Licensed and Regulated under the banner of the Gambling Commission, the casino has some of the best prices in the Malay market.
But does this casino have everything for a slot game lover? We will discover this in this thoroughly investigated article on the 918Kiss review. We will also show you how to download its APK, so stick with us.
What is 918Kiss?
918Kiss is a slot game platform with the highest popularity in the Malaysian market. You can play various slot games and engage with a vibrant gaming community on a few taps.
The hype of 918Kiss lies in providing opportunities to win money. They achieve this goal by not only providing high RTP games but also through other bonus features such as promotions, free credits, and jackpots. With 918Kiss, you can conveniently play games and earn money.
As a gambling enthusiast, I found the user interface pretty nice, and it was easy to shift between the different genres of slot games. All their games come with different reels and pay lines, and you always enjoy shuffling through the differently themed unique games.
Overall, 918Kiss is an excellent platform that always puts its customers first. With the highest popularity in Southeast Asia, they aim to dominate the world with their slot games.
Why 918Kiss is the preferred choice for gamers?
If you are asking whether 918Kiss is a preferred choice for gamers, let me tell you this. It is one of the best slot game providers out there, with numerous outstanding features to benefit from.
Firstly, a varied catalogue of games is available on the website. Secondly, 918Kiss ensures its security measures are strong enough to protect your account and data from possible threats or hacking attempts.
In case these are not convincing enough, there are other attractive features like being easy to navigate, getting gifts and credits, and having great chances for winning real cash prizes. Therefore, no matter what type of player you are, new or old, you can still win big prizes together in an honest community.
Slot Game Selection at 918Kiss
At 918Kiss, you will find hundreds of slot games to select from. We know settling into a slot game takes work, especially when crowded with a vast genre of games. That's why we present you with the top five slot games available at 918Kiss:
T-Rex
Its name gives you a general idea of what this game is based on. With the T-Rex slot game, you will experience a prehistoric theme and T-Rex wild symbols to trigger free spins. If you are looking for an immersive and entertaining companion, T-Rex might be the perfect candidate, as it comes with five reels and 25 25-pay line layouts.
PlayDirt
PlayDirt, on the other hand, is a game with multiple bonus features. It is a fan favourite in the 918 Kiss community because of its numerous chances of winning free spins. The game features a gold rush theme with lucrative offers to fill your pockets. The best thing about this game is its ability to trigger random jackpots anytime during the gameplay.
Highway Kings
Are you a highway games lover? So are we; that's why we found Highway Kings to be the most underrated slot game of 918Kiss. It has an attractive design with a layout system based on a truckers theme. We recommend checking Highway Kings at least once if you want the best. Here, you will see five reels and nine pay lines with an impressive RTP of 97.06%.
Wukong
In Chinese literature, Wukong is a game for lovers of the mythical figure Monkey King, also known as Sun Wukong. The game has three rows with 25 pay lines. The fewer the rows, the better the chance of landing wins. If you want prosperity blessed by the hands of Monkey King, you can check out Wukong in 918Kiss.
Bonus Bears
What does the name describe? A jungle-based reel filled with bears, bees, and honey symbols. This is what you get with Bonus Bears, along with a maximum payout of 5,000 times the bet per line.
For beginners, we recommend starting with the top five slot games of 918Kiss. However, if you are looking for a long list of what types of slot games you can expect from 918Kiss, here is a list:
- Cherry Love
- PayDirt
- Football
- SCR slot game
- Bonus Endure Dolphin Reef
- Blue
- Fortune Panda
- Robin Hood and the list goes on.
Tips to Win 918Kiss Slot Games
No one can guarantee you will win every game. But there are ways to maximise your chances of landing big wins – At least landing any win on the pay line. To successfully launch spin attacks on 918Kiss, please play according to these rules:
Set a Limit
If you don't want to spend more than you can afford, you need to limit how much you will lose to get a win.
Bet The Maximum
Betting on the maximum pay lines will increase the betting amount but unlock far more pay lines. In simple words, the probability of landing any combination will significantly increase.
Start With Lower Jackpots
One thing you should remember is that slot games with higher jackpots mean there are fewer chances of winning the game. In other words, lower jackpots mean an easy difficulty level and higher jackpots mean a hard one. As a beginner, always settle for slot games with lower jackpots.
How to Download 918Kiss On Your Mobile?
918Kiss works on both Androids and iOS devices, which means players can access online casino games from anywhere. So there is no need to wait anymore; here is how you can download 918Kiss on your phone.
For Android Users
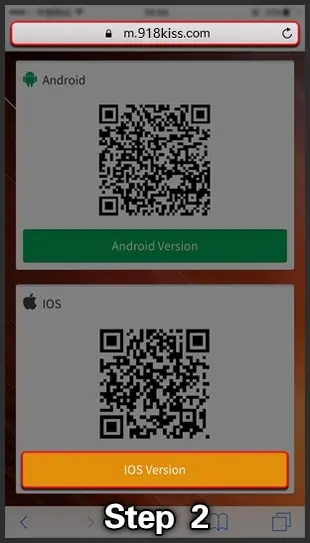
- To get this APK, you can download it from their official website or click here to start downloading the official APK via our direct link.
- Go to your Android's file manager and find the downloaded file after the download is finished.
- If you are unsure how to find it, click the install button and see if 918Kiss appears as an icon on your home screen.
- If it does not work, like being blocked for security reasons, etc., try allowing unknown sources in settings before installing any apps outside the Play Store.
For iOS Users
- Two types of APKs can be downloaded if you use an iOS device.
- If you own an iPhone 5 or higher, you must download the 64-bit app. However, if your device model is lower than 5, please download the 32-bit app.
- Open the downloaded file and click on the Install button after downloading it.
- Your device will prompt "Untrusted Enterprise Developer" when you try to open the game from your home screen.
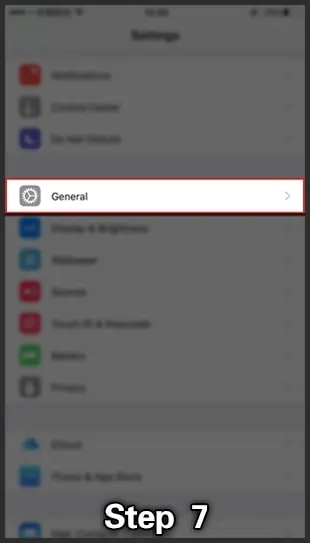
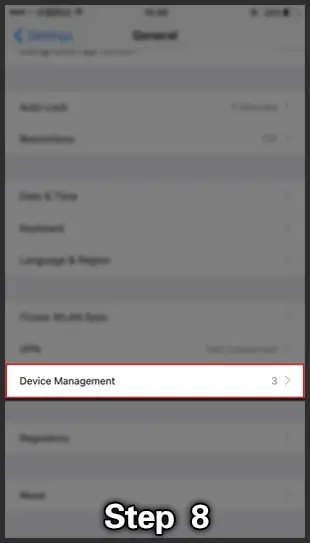
- Don't worry about that; go into Settings> General -> Device Management & find where they provide settings related to 918Kiss.
- Allow the app from the settings, and you are good to go.
How to Register and Start Playing at 918Kiss?
Installing the game is only helpful if registered with the 918Kiss platform. Don't worry; getting registered is easy, and you will unlock many slot games in your arsenal. But to do that, you must follow the guidelines given below:
- You can register by visiting the official website or opening the downloaded 918Kiss application.
- Once you open the app, you will be asked to enter your login credentials.
- If you are a new user, then look for the signup button to get registered.
- On the next page, you must enter the following details: username, email, full name, password, and mobile number. You may need to clear a Captcha depending on your network and region.
- After clicking the signup button, you will get registered with 918Kiss. But there is one last step.
- You must make a first deposit to verify your account to unlock all the fun and games.
Is 918Kiss Safe to Play?
When looking for a slot platform to settle with, almost every person checks the security measures to know they are communicating with a trustworthy platform. But how do you know if a platform is safe to play?
You look for different aspects, such as safe transactions, privacy policies, game variety, reviews, and reputation around the block. Luckily, 918Kiss thrives in providing these features.
With a strongly backed-up privacy policy, safe transactions, and 24/7 customer support, 918Kiss is one of the most trustworthy platforms. It follows robust security measures to protect its users from scams to ensure a quality gaming experience.
Is 918Kiss a Reliable Slot Game Platform?
Finding out if a platform is reliable is always a best practice before playing for real money. Is 918Kiss then a reliable platform? From emphasising safety and security, 918Kiss has proved to be a platform. This app prevents malware and virus threats to the user through 128-bit encryption and ensures that the user's data remains private. To keep your transactions safe, SSL Protection has been used by 918Kiss, which helps securely transfer data between your device and server.
918kiss APK Free Download for iOS and Android [New]
By Tan Sri Lim
Download 918Kiss APK for top online slot games. Easy, secure setup. Enhance your gaming adventure today!Requires : ANDROID,IOS,WINDOWS